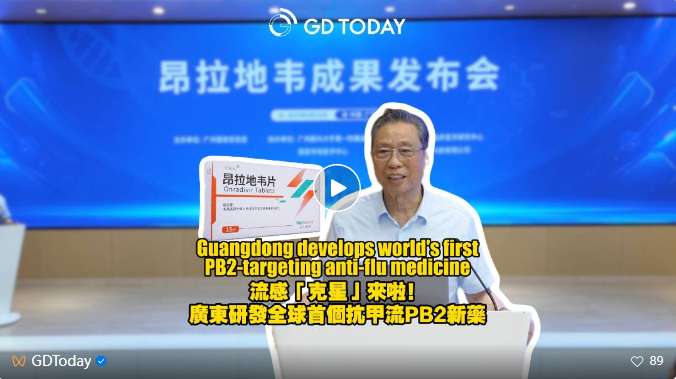
Guangdong develops world's first PB2-targeting anti-flu medicine
The Guangzhou National Laboratory has spearheaded the development of Onradivir tablets,the world's first innovative drug targeting the PB2subunit of the influenza A virus RNA polymerase,which demonstrates a40%faster reduction in symptoms in clinical trials compared to placebo.
The drug has been approved for marketing by China's National Medical Products Administration in May.
Significant efficacy
Relevant researchers indicate that Onradivir boasts remarkable advantages of rapid action,strong potency,and low resistance,promising to elevate the overall level of influenza prevention and treatment in China and globally.
Research data reveal that after taking Onradivir,patients experience a rapid decline in viral load.In vitro antiviral activity studies show a nearly40%reduction in symptom relief time compared to the placebo group(untreated group).In terms of fever reduction,the time is shortened by nearly39%compared to the placebo group.
Notably,Onradivir exhibits a low rate of resistant mutations,with no suspected resistance cases in Phase II clinical trials and a1.6%incidence in Phase III.It is also effective against"super flu viruses"resistant to some other marketed anti-influenza drugs.
Furthermore,Onradivir offers"multi-purpose"benefits,being effective against highly pathogenic avian influenza viruses such as H7N9and H5N6,thus providing a tool for responding to avian-to-human influenza outbreaks.
Providing a Chinese solution for global influenza control
Globally,there are approximately1billion influenza cases annually,including3to5million severe cases and290,000to650,000deaths.The influenza season during winter and spring imposes a significant public health burden worldwide.
Onradivir,as a new drug targeting the PB2subunit,employs a"cap-snatching"mechanism(seizing the mRNA cap of host cells),distinct from Baloxavir's"endonuclease cleavage"mechanism(cutting host mRNA).Previous attempts by foreign institutions to develop drugs using this mechanism did not achieve final success in clinical studies.
As the world's first innovative drug targeting the PB2subunit,Onradivir tablets offer a novel target for drug-resistant viruses,representing a Class1innovative drug of international standard from China.
The drug has been granted patent authorizations in multiple countries,including China and the United States.Preclinical basic pharmacological studies,as well as results from Phase II and III clinical trials,have been published in journals such as Drugs,The Lancet Infectious Diseases,and The Lancet Respiratory Medicine.
Zhong Nanshan,Academician of the Chinese Academy of Engineering and Director of the Guangzhou National Laboratory,stated that Onradivir's contribution lies not only in breakthroughs in drug efficacy and safety but also in providing a Chinese solution for global influenza control.
"Now we are very confident that it can be better than other international drugs and even more affordable,"said Zhong.
 最新热点
最新热点
-
遇见广州,解锁未来都市的N种模样 | 粤见APEC
-
独家视频丨习近平同乌拉圭总统奥尔西举行会谈
-
独家视频丨习近平同乌拉圭总统会谈:持续深化全面战略伙伴关系 加强全球南方团结协作
独家视频丨习近平同乌拉圭总统会谈:持续深化全面战略伙伴关系 加强全球南方团结协作
最新热点2月3日上午,国家主席习近平在北京人民大会堂同来华进行国事访问的乌拉圭总统奥尔西举行会谈。 习近平指出,中国有句古语,“相知无远近,万...
-
独家视频丨习近平同乌拉圭总统会谈:你是中国人民的好朋友 也是今年首位访华的拉美国家元首
独家视频丨习近平同乌拉圭总统会谈:你是中国人民的好朋友 也是今年首位访华的拉美国家元首
最新热点2月3日上午,国家主席习近平在北京人民大会堂同来华进行国事访问的乌拉圭总统奥尔西举行会谈。 习近平指出,38年前的今天,中乌实现建交。38年来,无...
-
广东这个千年古县,韩愈来了都说好















 北京将打造现代化首都都市圈 壮大先进制造业集群
北京将打造现代化首都都市圈 壮大先进制造业集群
 中国“个性年货”俏销 多元供给精准对接需求
中国“个性年货”俏销 多元供给精准对接需求
 中国成功发射卫星互联网低轨18组卫星
中国成功发射卫星互联网低轨18组卫星
